- Home Page
- Company Profile
-
Our Products
- Pharmaceutical Tablets
- Vitamin D3 Tablets
- Clotrimazole Tablet
- Clomifene Tablets BP 50mg
- Ciprofloxacin Tablets 500mg
- Sulfadiazine Tablet
- Sodium Bicarbonate Tablets
- Simvastatin Tablets
- Cefuroxime Axetile Tablets
- Nystatin Vaginal Inserts
- VITAMIN B COMPLEX TABLETS
- Ferrous Sulphate And Folic Acid Tablet
- Nystatin Tablets
- Artemether and Lumefantrine Tablets
- Colloidal bismuth subcitrate 120 mg tablets
- Primaquine Tablets
- Nifedipine Extended Release Tablets USP 20 mg
- Quinine Sulphate Tablet 300 mg
- Warfarin Tablets
- Calcium Folinato
- Tinidazole Tablets
- Fluconazole Tab
- Fexofenadine HCL Tablets
- Artemether & Lumefantrine Tab
- Aceclofenac & Diacerein Tablets
- Colchicine Tablets
- Amodiaquine Tablets
- Ferrous Sulphate and Folic Acid
- Prednisolone Tablets
- Prochlorperazine Tablets
- MIstop Talets
- ENALAPRIL MALEATE TABLETS USP 5MG
- Doxycyclin Hyclate Tablets USP 100 mg
- Calcium With Vitamin D3 Chewable Tablets
- Dapsone Tablets BP 50 Mg
- Albendazole Tablets 400 Mg
- Erythromycin Stearate Tablets BP
- Aminophylline 100mg Tablets
- Fluoxetine Tablet 20mg
- Cefixime Tablets USP 200 mg
- Sulfadoxine Pyrimetanine Tablet
- Lisinopril Hydrochlorothiazide tablets USP
- Ibuprofen Tablets 400mg
- Cefixime Tablets USP 400 mg
- Carvedilol Tablets
- Cefalexin Capsules BP 250mg
- Ciprofloxacin Tablets USP 750 mg
- Bisacodyl Tablets
- Prednisolone Tablets USP 20 mg
- Nitrofurantoin Tablets BP
- Amitriptyline Tablets
- Amoxicillin Dispersible Tablets
- Ciprofloxacin Tablets USP 500
- Chloroquine Phosphate Tablets BP
- ALBENDAZOLE TABLETS USP 200mg
- OLANZAPINE 10mg
- Prolonged - Release Potassium Chloride Tablets BP
- Ciprofloxacin Tablets USP 500mg
- Ciprofloxacin Tablets USP 500 mg
- Senna Tablets
- Gastro-resistant Diclofenac Tablets 50mg
- Clopidogrel Tablets USP
- ClOPIDOGREL TABLETS USP 75mg
- Ranitidine Tablets USP
- ALUMINIUM HYDROXIDE TABLETS BP 500mg
- Metronidazole Tablets BP 500mg
- Lisinopril Tablets USP 10mg
- Paracetamol Phenylephrine Hydrochloride and Chlorpheniramine Maleate Tablets
- Pyrimethamine Tablet
- Albendazole Tablets
- Carvedilol Tablets USP
- Carbimazole Tablets
- Lisinopril Tablets
- Prednisolone Tablets BP
- Griseofulvin Tablets BP
- Phenytoin Tablets BP
- Norfloxacin Tablets BP
- Ranitidine Tablet
- Levofloxacin Tablets
- Esomeprazole Tablets
- Devazole Tablet
- Fluconazole Tablet
- Paracetamol Phenylephrine HCl and Chlorpheniramine Maleate Tablets
- CLOTRIMAZOLE VAGINAL PESSARY
- Nifedipine Extended Release Tablets
- Betahistine Dihydrochloride Tablets
- Chloroquine Phosphate
- Azithromycin Tablets
- Dapsone Tablets
- sofosbuvir tablets
- Rivadev Tablet
- Devkabir Tablet
- Koldavin T
- Chloroquine Phosphate Tablets
- Devpantop
- Sevlamer HYdrochloride Tablets
- Alfuzosin Prolonged Release Tablets
- Methyldopa Tablets BP
- Zinc Sulfate Tablets USP 20 mg
- Gastro Resistant Sodium Valproate Tablets BP 200mg
- Praziquantel Tablets USP 600 mg
- Salbutamol Tablets BP 2 mg
- Daclatasvir 60 mg & Sofosbuvir 400 mg Tablets
- Enlekavir Tablets USP 0.5 mg
- Clomifene Tablets BP 50 mg
- Leflunomide Tablets BP 10 mg
- Pharmaceutical Injections
- DEVCEF 1000 (Ceftriaxone for Injection USP 1000 mg)
- Progesterone injection
- Prednisolone Acetate Injectable Suspension USP
- Sterilised Water for Injection
- Iron Sucrose Injection
- Bupivacaine Hcl with Dextrose Injection USP
- Adrenaline Injection
- Atenolol Injection
- Bupivacaine Injection
- Calcium Gluconate Injection
- Ceftriaxone Injection
- Chlorpromazine Injection
- Cloxacillin Injection
- Dobutamine Injection
- Kanamycin Injection
- Lidocaine Injection
- Phenytoin Sodium Injections
- Promethazine Injection
- Magnesium Sulfate Injection USP 200mg
- Andrenaline Inj. BP
- Potassium Chloride for Injection Concentrate USP 100 mg/mL
- HEPARIN SODIUM INJECTION BP 5000 UI/ML
- Dopamine Hydrochloride Injection USP 200mg
- Clindamycin Injection
- CLINDAMYCIN INJECTION USP 150mg/ml
- Gentamycin Sulphate Injection
- Medroxyprogesterone Acetate Injection
- Dobutamine Injection USP 12.5 mg/mL
- Iron Dextran Injections
- Devoxa Injection
- Bupivacaine hcl in dextrose injection USP
- Pantoprazole For Injection 40mg
- Vancomycin Hcl Injection
- Imipramine HCL Injection
- Ceftriaxone For Injection USP 1g
- Suxamethonium Chloride Injection BP 50 mg/mL
- Vondan Ondansetron Injection
- Adrenaline Injection BP 1mg
- Vancomycin Hydrochloride For Injection
- Magnesium Sulfate Injection USP 500mg
- Hydralazine HCL Injection
- Noradrenaline Injection
- Potassium Chloride Injection
- Clonidine HCL Injection
- Amphotericin B
- Methylcobalamine Injection
- Sterile Potassium Chloride Concentrate
- Phenytoin Sodium Injection
- Ranitidine Injections
- Furosemide Injection
- Ondansetron Injection
- Ranitidine Injection
- Creams and Ointments
- Betamethasone Valerate + Gentamicin Sulphate+Tonaftate Iodochlorhydroxyquinoline Cream
- Povidone Iodine Ointment U.S.P
- Metronidazole with Povidone Iodine Cream
- Lidocaine Jelly
- Crotamiton Cream
- Miconazole cream BP
- Hydroquinone Cream
- Silver Sulphadiazine Cream
- Diclofenac Sodium Gel
- Tretinoin Cream
- Clobetasole Propionate Cream
- Fusidic Acid
- Acyclovir Ointment
- Capsaicin Gel
- Betamethasone Dipropionate Clotrimazole Neomycin Cream
- Anti-Hemorrhoidal Ointment 10 g
- Anti-Hemorrhoidal Ointment 20 g
- Zinc Oxide and Castor Oil Cream
- Beclomethasone Clotrimazole Neomycin Cream
- Beclomethasone / Clotrimazole / Neomycin Cream
- Capsin Gel
- Khwaderm Cream
- Miconazole Nitrate Cream
- Acyclovir Ointments
- Hard Gelatin Capsules
- Amoxicillin Capsules BP 500mg
- FLUCLOXACLLIN CAPSULES BP 500mg
- Cefalexin Capsules BP 500mg
- Cefuroxime Axetile Tablets USP 250mg
- Fluconazole Capsules 200 mg
- Doxycycline Capsules BP 100mg
- Esomeprazole 20mg
- Esomeprazole 40mg
- Fluconazole Capsules
- Methylcobalamin and Pregabalin Capsules
- Azithromycin Capsules
- Oral Suspension
- Ibuprofen and Paracetamol Suspension
- Azithromycin for oral suspension USP
- Cefixime for Oral Suspension USP 100 mg/5 mL
- Artemether and Lumefantrine Oral Suspension
- Ciprofloxacin Suspension
- Carbocisteine Suspension
- Peadiatric Multivitamin Drops
- Artemether and Lumefantrine For Oral Suspension
- Amoxicillin Oral Suspension BP 250 mg
- Fluconazole Oral Suspension 50mg/5ml
- Artemether & Lumefantrine 180/1080
- Azithromycin For Oral Suspension USP
- Cefixime For Oral Suspension USP
- Metronidazole Oral suspension BP 125mg/5ml
- Bromhexine Hydrochloride
- Cloxacillin Sodium
- Quinine Sulfate
- Bromhexine Hydrochloride Elixir
- Dr. Mol Paracetamol Oral Suspension
- Devalzyn Suspension
- Liquid Orals
- Soft Gelatin Capsules
- Injection Large Volume Parenterals
- Pharmaceutical Capsules
- Dry Syrup
- Eye Drops / Ear Drops
- Antiseptic & Antiparasitic Lotions
- Pharmaceutical Tablets
- More Info
- Contact Us
Ceftriaxone For Injection USP 1g
Ceftriaxone For Injection USP 1g Specification
- Drug Type
- General Medicines
- Ingredients
- COMPOSITION: Each vial contains: Ceftriaxone Sodium USP Eq. to Ceftriaxone .......... 1g
- Dosage
- AS RECOMMENDED BY PHYSICIAN
- Dosage Guidelines
- AS RECOMMENDED BY PHYSICIAN
- Storage Instructions
- Store at Room Temperature away from heat and light. Keep out of reach of children.
Ceftriaxone For Injection USP 1g Trade Information
- Sample Available
- Yes
- Packaging Details
- Single Vial with 10ml WFI in tray with printed carton with pack insert.
About Ceftriaxone For Injection USP 1g
DEVCEF 1000
Ceftriaxone For Injection USP 1g
COMPOSITION:
Each vial contains:
Ceftriaxone Sodium USP Eq. to Ceftriaxone ..........1g
Pharmacotherapeutic group
J01DA13 - Cephalosporins and related substances.
Pharmacodynamic effects : Ceftriaxone exerts in vitroactivity against a wide range of Gram-negative and Gram-positivemicroorganisms. Ceftriaxone is highly stable to most beta-lactamases, both thepenicillinases and cephalosporinases, of Gram-positive and Gram-negativebacteria.
Antibiotic class : Long acting, broad spectrumsemi-synthetic cephalosporin for intramuscular or intravenous administration.
Antibiotic nature and mode of action : Thebactericidal activity of ceftriaxone results from inhibition of bacterial cellwall synthesis.
Indications: Infections caused by pathogens sensitiveto Ceftriaxone Injection, e.g.: sepsis; meningitis; abdominal infections(peritonitis, infections of the biliary and gastrointestinal tracts);infections of the bones, joints, soft tissue, skin and of wounds; infections inpatients with impaired defence mechanisms; renal and urinary tract infections;respiratory tract infections, particularly pneumonia, and ear, nose and throatinfections; genital infections, including gonorrhoea. Perioperative prophylaxisof infections.
Product details
|
Shelf life |
2 Years |
|
Also gives |
Third Party Manufacturing |
|
Country of Origin |
Made in India |
|
Treatment |
Antibiotics |
|
Packaging Type |
Glass Ampoules |
|
Composition |
Each vial contains |
|
: Form |
Injection |
|
Brand Name |
DEVCEF 1000 |
|
Dosage |
As directed by the physician. |
|
Injection Site |
IV & IM |
|
Manufacturer |
DEVLIFE CORPORATION PVT. LTD. |
|
Strength |
1 gm |
FAQs of Ceftriaxone For Injection USP 1g:
Q: What is the drug type of Ceftriaxone For Injection USP 1g?
A: The drug type of Ceftriaxone For Injection USP 1g is General Medicines.Q: What are the ingredients in Ceftriaxone For Injection USP 1g?
A: Each vial contains Ceftriaxone Sodium USP equivalent to 1g of Ceftriaxone.Q: What is the recommended dosage for Ceftriaxone For Injection USP 1g?
A: The dosage should be as recommended by a physician.Q: How should Ceftriaxone For Injection USP 1g be stored?
A: It should be stored at room temperature away from heat and light, and kept out of reach of children.Q: Are there specific dosage guidelines for Ceftriaxone For Injection USP 1g?
A: The dosage guidelines should be followed as recommended by a physician.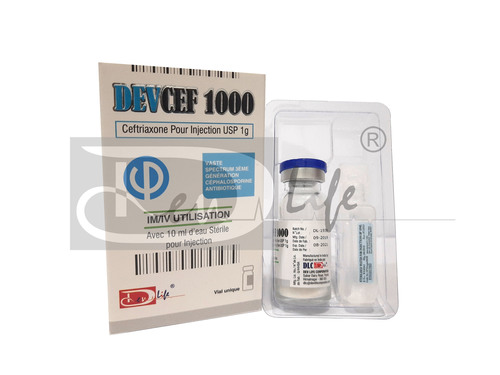
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Injections Category
Bupivacaine Injection
Physical Form : Other, Injection
Drug Type : Anesthetic, Other
Storage Instructions : Store below 25C protect from light and keep out of reach of children.
Origin of Medicine : Synthetic
Dosage Guidelines : Administered by a healthcare provider; dose not to exceed maximum recommended amount to avoid toxicity.
Promethazine Injection
Physical Form : Other, Injection
Drug Type : Other, Antihistamine
Storage Instructions : Store at 2025C (6877F) and protect from light
Origin of Medicine : Synthetic
Dosage Guidelines : Administer intramuscularly or intravenously as prescribed by a healthcare professional
Vondan Ondansetron Injection
Minimum Order Quantity : 10 Pieces
Physical Form : Liquid
Drug Type : Injection
Storage Instructions : Cool & Dry Place
Origin of Medicine : India
Dosage Guidelines : As directed by the Physician.
Progesterone injection
Minimum Order Quantity : 10 Pieces
Physical Form : Liquid
Drug Type : Injection
Storage Instructions : Cool & Dry Place
Origin of Medicine : India

 Send Inquiry
Send Inquiry