- Home Page
- Company Profile
-
Our Products
- Pharmaceutical Tablets
- Sulfadiazine Tablet
- Nystatin Vaginal Inserts
- Simvastatin Tablets
- Clotrimazole Tablet
- Clomifene Tablets BP 50mg
- Ciprofloxacin Tablets 500mg
- Sodium Bicarbonate Tablets
- Ferrous Sulphate And Folic Acid Tablet
- Nystatin Tablets
- Colloidal bismuth subcitrate 120 mg tablets
- Primaquine Tablets
- Cefuroxime Axetile Tablets
- Prednisolone Tablets
- Vitamin D3 Tablets
- Prochlorperazine Tablets
- Ferrous Sulphate and Folic Acid
- Amodiaquine Tablets
- Colchicine Tablets
- Aceclofenac & Diacerein Tablets
- Artemether & Lumefantrine Tab
- Fexofenadine HCL Tablets
- Fluconazole Tab
- Tinidazole Tablets
- Calcium Folinato
- Warfarin Tablets
- Quinine Sulphate Tablet 300 mg
- Nifedipine Extended Release Tablets USP 20 mg
- Artemether and Lumefantrine Tablets
- VITAMIN B COMPLEX TABLETS
- ENALAPRIL MALEATE TABLETS USP 5MG
- MIstop Talets
- Doxycyclin Hyclate Tablets USP 100 mg
- Calcium With Vitamin D3 Chewable Tablets
- Dapsone Tablets BP 50 Mg
- Albendazole Tablets 400 Mg
- Fluoxetine Tablet 20mg
- Aminophylline 100mg Tablets
- Erythromycin Stearate Tablets BP
- Lisinopril Hydrochlorothiazide tablets USP
- Cefixime Tablets USP 200 mg
- Ibuprofen Tablets 400mg
- Sulfadoxine Pyrimetanine Tablet
- Cefixime Tablets USP 400 mg
- Carvedilol Tablets
- Cefalexin Capsules BP 250mg
- Ciprofloxacin Tablets USP 750 mg
- Prednisolone Tablets USP 20 mg
- Bisacodyl Tablets
- Amitriptyline Tablets
- Nitrofurantoin Tablets BP
- Ciprofloxacin Tablets USP 500
- Chloroquine Phosphate Tablets BP
- Amoxicillin Dispersible Tablets
- Prolonged - Release Potassium Chloride Tablets BP
- Ciprofloxacin Tablets USP 500mg
- ALBENDAZOLE TABLETS USP 200mg
- OLANZAPINE 10mg
- Gastro-resistant Diclofenac Tablets 50mg
- Senna Tablets
- Ciprofloxacin Tablets USP 500 mg
- Clopidogrel Tablets USP
- ClOPIDOGREL TABLETS USP 75mg
- ALUMINIUM HYDROXIDE TABLETS BP 500mg
- Metronidazole Tablets BP 500mg
- Ranitidine Tablets USP
- Lisinopril Tablets USP 10mg
- Paracetamol Phenylephrine Hydrochloride and Chlorpheniramine Maleate Tablets
- Pyrimethamine Tablet
- Albendazole Tablets
- Carvedilol Tablets USP
- Carbimazole Tablets
- Lisinopril Tablets
- Prednisolone Tablets BP
- Griseofulvin Tablets BP
- Phenytoin Tablets BP
- Norfloxacin Tablets BP
- Ranitidine Tablet
- Levofloxacin Tablets
- Esomeprazole Tablets
- Devazole Tablet
- Fluconazole Tablet
- Paracetamol Phenylephrine HCl and Chlorpheniramine Maleate Tablets
- CLOTRIMAZOLE VAGINAL PESSARY
- Nifedipine Extended Release Tablets
- Betahistine Dihydrochloride Tablets
- Chloroquine Phosphate
- Azithromycin Tablets
- Dapsone Tablets
- sofosbuvir tablets
- Rivadev Tablet
- Devkabir Tablet
- Koldavin T
- Chloroquine Phosphate Tablets
- Devpantop
- Sevlamer HYdrochloride Tablets
- Alfuzosin Prolonged Release Tablets
- Methyldopa Tablets BP
- Zinc Sulfate Tablets USP 20 mg
- Gastro Resistant Sodium Valproate Tablets BP 200mg
- Praziquantel Tablets USP 600 mg
- Salbutamol Tablets BP 2 mg
- Daclatasvir 60 mg & Sofosbuvir 400 mg Tablets
- Enlekavir Tablets USP 0.5 mg
- Clomifene Tablets BP 50 mg
- Leflunomide Tablets BP 10 mg
- Epiryte 500
- Pantoprazole Gastroresistant tablets
- Chloroquine Phosphate Tablets
- Pharmaceutical Injections
- Chlorpromazine Injection
- Dobutamine Injection
- Lidocaine Injection
- Promethazine Injection
- Phenytoin Sodium Injections
- Kanamycin Injection
- Progesterone injection
- Prednisolone Acetate Injectable Suspension USP
- Sterilised Water for Injection
- Iron Sucrose Injection
- Bupivacaine Hcl with Dextrose Injection USP
- DEVCEF 1000 (Ceftriaxone for Injection USP 1000 mg)
- Cloxacillin Injection
- Adrenaline Injection
- Atenolol Injection
- Bupivacaine Injection
- Calcium Gluconate Injection
- Ceftriaxone Injection
- Magnesium Sulfate Injection USP 200mg
- Andrenaline Inj. BP
- Potassium Chloride for Injection Concentrate USP 100 mg/mL
- HEPARIN SODIUM INJECTION BP 5000 UI/ML
- Dopamine Hydrochloride Injection USP 200mg
- Clindamycin Injection
- CLINDAMYCIN INJECTION USP 150mg/ml
- Gentamycin Sulphate Injection
- Medroxyprogesterone Acetate Injection
- Dobutamine Injection USP 12.5 mg/mL
- Iron Dextran Injections
- Devoxa Injection
- Bupivacaine hcl in dextrose injection USP
- Vancomycin Hcl Injection
- Pantoprazole For Injection 40mg
- Imipramine HCL Injection
- Ceftriaxone For Injection USP 1g
- Suxamethonium Chloride Injection BP 50 mg/mL
- Vondan Ondansetron Injection
- Vancomycin Hydrochloride For Injection
- Adrenaline Injection BP 1mg
- Magnesium Sulfate Injection USP 500mg
- Hydralazine HCL Injection
- Noradrenaline Injection
- Potassium Chloride Injection
- Clonidine HCL Injection
- Amphotericin B
- Methylcobalamine Injection
- Sterile Potassium Chloride Concentrate
- Phenytoin Sodium Injection
- Ranitidine Injections
- Furosemide Injection
- Ondansetron Injection
- Ranitidine Injection
- Creams and Ointments
- Diclofenac Sodium Gel
- Betamethasone Valerate + Gentamicin Sulphate+Tonaftate Iodochlorhydroxyquinoline Cream
- Povidone Iodine Ointment U.S.P
- Metronidazole with Povidone Iodine Cream
- Lidocaine Jelly
- Crotamiton Cream
- Miconazole cream BP
- Hydroquinone Cream
- Silver Sulphadiazine Cream
- Tretinoin Cream
- Clobetasole Propionate Cream
- Fusidic Acid
- Acyclovir Ointment
- Capsaicin Gel
- Betamethasone Dipropionate Clotrimazole Neomycin Cream
- Anti-Hemorrhoidal Ointment 10 g
- Anti-Hemorrhoidal Ointment 20 g
- Zinc Oxide and Castor Oil Cream
- Beclomethasone Clotrimazole Neomycin Cream
- Beclomethasone / Clotrimazole / Neomycin Cream
- Capsin Gel
- Khwaderm Cream
- Miconazole Nitrate Cream
- Acyclovir Ointments
- Hard Gelatin Capsules
- Amoxicillin Capsules BP 500mg
- FLUCLOXACLLIN CAPSULES BP 500mg
- Cefalexin Capsules BP 500mg
- Cefuroxime Axetile Tablets USP 250mg
- Fluconazole Capsules 200 mg
- Doxycycline Capsules BP 100mg
- Esomeprazole 20mg
- Esomeprazole 40mg
- Fluconazole Capsules
- Methylcobalamin and Pregabalin Capsules
- Azithromycin Capsules
- Oral Suspension
- Ibuprofen and Paracetamol Suspension
- Azithromycin for oral suspension USP
- Cefixime for Oral Suspension USP 100 mg/5 mL
- Artemether and Lumefantrine Oral Suspension
- Ciprofloxacin Suspension
- Carbocisteine Suspension
- Peadiatric Multivitamin Drops
- Artemether and Lumefantrine For Oral Suspension
- Amoxicillin Oral Suspension BP 250 mg
- Fluconazole Oral Suspension 50mg/5ml
- Artemether & Lumefantrine 180/1080
- Azithromycin For Oral Suspension USP
- Cefixime For Oral Suspension USP
- Metronidazole Oral suspension BP 125mg/5ml
- Bromhexine Hydrochloride
- Cloxacillin Sodium
- Quinine Sulfate
- Bromhexine Hydrochloride Elixir
- Dr. Mol Paracetamol Oral Suspension
- Devalzyn Suspension
- Liquid Orals
- Soft Gelatin Capsules
- Injection Large Volume Parenterals
- Pharmaceutical Capsules
- Dry Syrup
- Eye Drops / Ear Drops
- Antiseptic & Antiparasitic Lotions
- Pharmaceutical Tablets
- More Info
- Contact Us
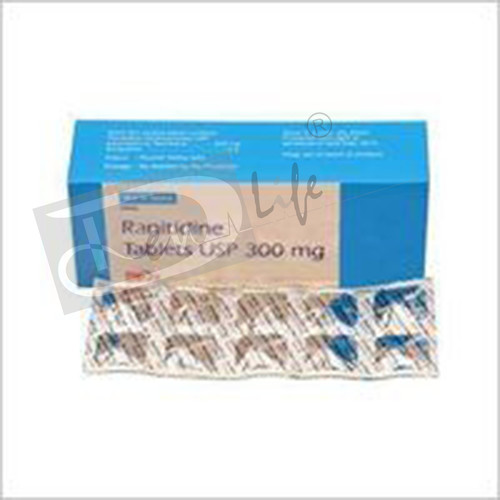
Ranitidine Tablets USP
MOQ : 10 Pieces
Ranitidine Tablets USP Specification
- Origin of Medicine
- India
- Salt Composition
- Ranitidine
- Drug Type
- General Medicines
- Ingredients
- Each film coated tablet contains: Ranitidine Hydrochloride USP Eq. to Ranitidine.150mg Excipientsq.s Colour: Sunset Yellow Lake
- Physical Form
- Tablets
- Dosage
- 150mg
- Suitable For
- Children
- Storage Instructions
- Dry Place
About Ranitidine Tablets USP
Each film coated tablet contains:
Ranitidine Hydrochloride USP
Eq. to Ranitidine 150mg
Excipients q.s
Colour: Sunset Yellow Lake
INDICATION:
Adults
GASTRISUREtablets are indicated for the treatment of benign gastric ulcers and duodenalulcers, including that associated with non-steroidal anti-inflammatory agents.
Preventionof non-steroidal anti-inflammatory drug associated duodenal ulcers, especiallyin patients with a history of peptic ulcer disease.
Treatmentof duodenal ulcers associated withHelicobacterpyloriinfection.
Post-operativeulcer.
Oesophagealreflux disease including long term management of healed oesophagitis.
Symptomaticrelief in gastro-oesophageal reflux disease.
Zollinger-EllisonSyndrome
Chronicepisodic dyspepsia, characterised by pain which is related to meals or disturbssleep but not associated with the above conditions.
Prophylaxisof gastrointestinal haemorrhage from stress ulceration in seriously illpatients
Prophylaxisof recurrent haemorrhage with bleeding peptic ulcers.
Beforegeneral anaesthesia in patients at risk of acid aspiration, particularlyobstetric patients during labour.
Ranitidinetablets are indicated for the long-term treatment of duodenal and benigngastric ulcers to prevent their recurrence. Long-term treatment is indicated inpatients with a history of recurrent ulcers.
Children(3 to 18 years)
Short term treatment of peptic ulcer
Treatment of gastro-oesophageal reflux, including reflux oesophagitis andsymptomatic relief of gastro-oesophageal reflux disease
FAQs of Ranitidine Tablets USP:
Q: What is the dosage of Ranitidine Tablets USP?
A: The dosage of Ranitidine Tablets USP is 150mg.Q: Where is the origin of Ranitidine Tablets USP?
A: The origin of Ranitidine Tablets USP is India.Q: What is the salt composition of Ranitidine Tablets USP?
A: The salt composition of Ranitidine Tablets USP is Ranitidine.Q: What is the suitable age group for Ranitidine Tablets USP?
A: Ranitidine Tablets USP is suitable for children.Q: What is the storage instruction for Ranitidine Tablets USP?
A: Ranitidine Tablets USP should be stored in a dry place.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Tablets Category
Paracetamol Phenylephrine HCl and Chlorpheniramine Maleate Tablets
Minimum Order Quantity : 50000
Drug Type : General Medicines
Storage Instructions : Dry Place
Physical Form : Tablets
Dosage : As per suggestion
Phenytoin Tablets BP
Minimum Order Quantity : 10 Pieces
Drug Type : General Medicines
Storage Instructions : Dry Place
Physical Form : Tablets
Dosage : 100mg
Ingredients : Each film coated tablet contains: Phenytoin Sodium BP ......100 mg Excipients ............................ q.s. Colour : Titanium Dioxide BP
Ranitidine Tablet
Drug Type : Other, Antacid
Storage Instructions : Store in a cool dry place away from direct sunlight and out of reach of children.
Physical Form : Other, Tablet
Dosage : 150 mg or 300 mg per tablet
Ingredients : Ranitidine Hydrochloride
Clomifene Tablets BP 50mg
Drug Type : General Medicines
Storage Instructions : Store at Room Temperature away from heat and light. Keep out of reach of children.
Physical Form : Tablets
Dosage : AS RECOMMENDED BY PHYSICIAN
Ingredients : Each uncoated tablet contains: Clomifene Citrate BP .............. 50 mg Excipients .......................................... q.s.

 Send Inquiry
Send Inquiry